Thermal sterilization of food
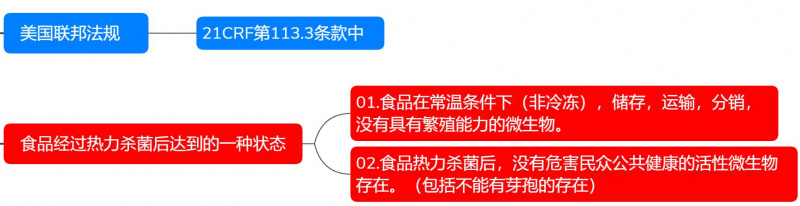
The concept of commercial sterility in food first appeared in Section 113.3 of Title 21 CFR (Code of Federal Regulations) in the United States. It refers to the state a food product reaches after undergoing thermal sterilization.
01. Food is stored, transported, and distributed under normal temperature conditions (non-frozen), without the presence of any microorganisms capable of reproduction.
02. After thermal sterilization, the food contains no viable microorganisms that could pose a threat to public health (including bacterial spores).
China issued its standard for testing commercial sterility in September 1989: GB4789.26-1989, which was later revised to GB/T4789.26-2003, titled "Microbiological Examination of Food Hygiene – Examination for Commercial Sterility of Canned Foods."

Commercial sterility does not mean absolute sterility; there may be bacteria present, but they must not be able to grow or reproduce, nor pose any health risk to consumers.
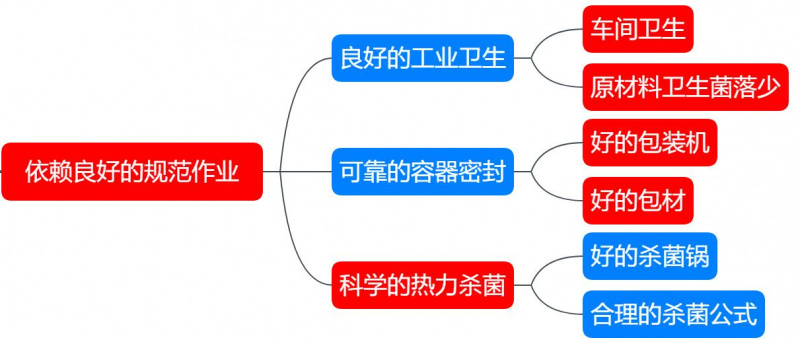
Good commercial sterility depends on proper operational practices, excellent industrial hygiene, reliable container sealing, and scientific thermal sterilization. These three factors are essential for achieving commercial sterility in food products.
Want to learn more? Click below:








